Probiotic food and food supplements have recently gained popularity as they contain beneficial microorganisms, typically bacteria and yeast, believed to have a positive impact on health when consumed in adequate amounts. These microorganisms have been incorporated into a wide range of products, with nutritional and health benefits such as improving gut health, digestion, the immune system. However, ‘probiotics’, in and of themselves, do not constitute specific health claims. Climate change and evolving consumer trends have redirected the EU´s attention toward new ways of producing and consuming food to improve its safety, quality and sustainability: probiotics microorganisms can offer a multifaceted contribution to innovation and sustainability in food systems.
Germany, France, the U.K., Italy and Spain are the leading European markets for probiotics. They represent approximately 61% of the European market of probiotic supplements. The retail value of the probiotic market (which includes sour milk products, probiotic yoghurts, and probiotic supplements) went from €8.6 billion in 2018 to €9.4 billion in 2022. E-In the meantime, online sales growth 2021 and 2022 for probiotic supplements is driven by Europe. The track of the online sales in 2022 shows that about 48% of products are from outside Europe labelled with probiotic claims. EU players are at a competitive disadvantage: probiotics suffer from uncertainty at the EU level linked to a restrictive regulatory approach to using the term ‘probiotics’. This led to the peculiar situation in which probiotics cannot be advertised as such on food labels in the EU market.
The majority of the panel of consumers do not feel well informed that a product contains probiotics (57% out of 8.000); when asked if they want to know more about the packaging and label, the percentage rises to 79%.
While the European market for food with beneficial live bacteria known as probiotics is ‘alive’, the EU regulatory framework is stuck in 2006. Requests to the European Commission from the European Deputies Parliament and from stakeholders for overcoming the outdated rules are piling up.In absence of a clear European framework, European countries are adopting national practices allowing the use of the term ‘probiotic’ on the label and communication, under conditions of use.
A consumer survey in 8 countries shows what consumers know and what they would like to know more about when it comes to probiotics. It also highlights that consumers would like to be better informed on the labelling and in communications about probiotic food, and probiotic microorganisms in food and food supplements. The survey was conducted in 8 European countries (Italy, Denmark, the Netherlands, Spain, Poland, Belgium, Germany, and Sweden) on 8000 consumers.
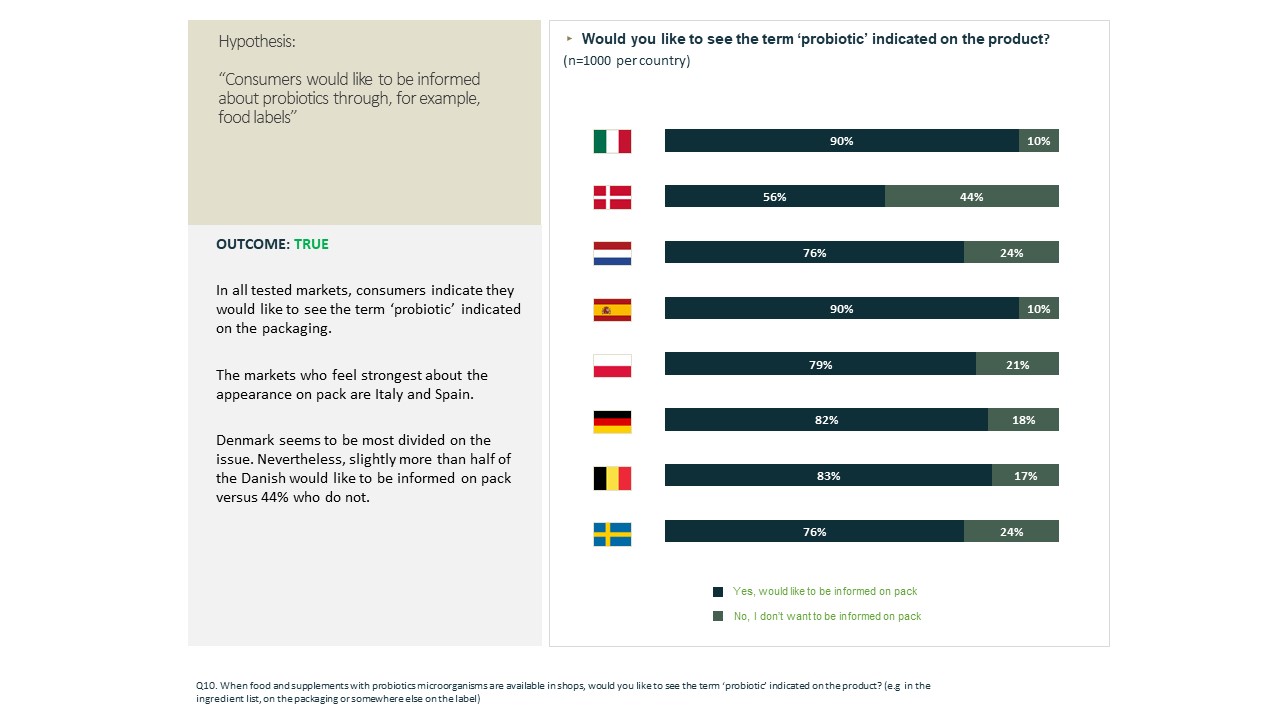
The majority of the panel of consumers do not feel well informed that a product contains probiotics (57% out of 8.000); when asked if they want to know more about the packaging and label, the percentage rises to 79% on average. On average, 79% would like to see the term ‘probiotic’ indicated on food and supplements with probiotics microorganisms (e.g. in the ingredient list, on the packaging or somewhere else on the label).
The EU should be responsive to innovation, while also providing regulatory predictability. The International Probiotics Association Europe (IPA Europe) is committed to promoting the responsible use of probiotics in food and food supplements. IPA Europe asks for an evaluation of the current understanding of the term ‘probiotic’ in the EU. Clear criteria should be set up to prevent the indiscriminate use of the term ‘probiotic’ and its potential misinterpretation (IPA Europe and ISAPP open-access paper “Criteria to qualify microorganisms as ‘probiotic’ in foods and dietary supplements) .

We request the support and collaboration of MEPs and relevant stakeholders in advancing this initiative. This proposal aligns with the broader goals of ensuring food safety, consumer protection, and the advancement of the European food industry.
Sign up to The Parliament's weekly newsletter
Every Friday our editorial team goes behind the headlines to offer insight and analysis on the key stories driving the EU agenda. Subscribe for free here.